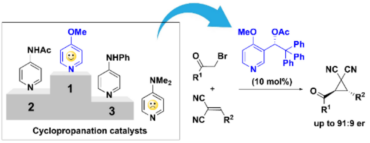
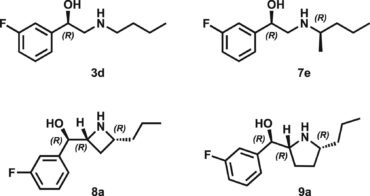
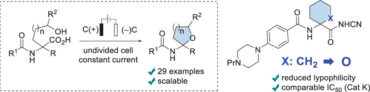
50. Development of novel β2-adrenergic receptor agonists for the stimulation of glucose uptake – The importance of chirality and ring size of cyclic amines
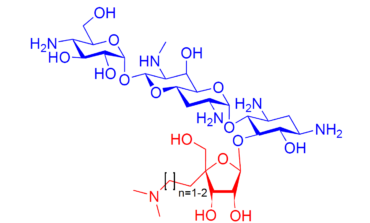
49. Synthesis and Evaluation of Novel 5-O-(4-C-Aminoalkyl-β-D-ribofuranosyl) Apramycin Derivatives for the Inhibition of Gram-Negative Pathogens Carrying the Aminoglycoside Phosphotransferase(3′)-Ia Resistance Determinant
DOI: 10.1002/hlca.202300138.
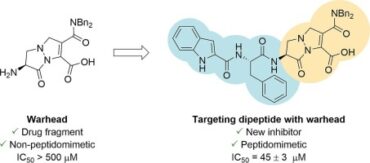
48. Pyrazolidinone-based peptidomimetic SARS-CoV-2 Mpro inhibitors
DOI: 10.1016/j.bmcl.2023.129530.
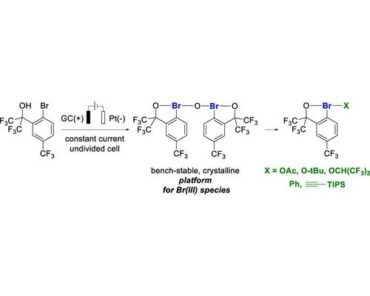
47. Electrochemical Synthesis of Unnatural Amino Acids via Anodic Decarboxylation of N-Acetylamino Malonic Acid Derivatives
DOI: https://doi.org/10.1021/acs.orglett.3c02687.
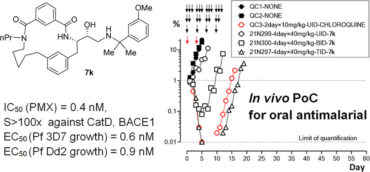
46. Macrocyclic Peptidomimetic Plasmepsin X Inhibitors with Potent In Vitro and In Vivo Antimalarial Activity
DOI: https://doi.org/10.1021/acs.jmedchem.3c00812.
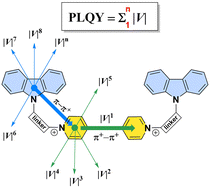
45. The emission efficiency of cationic solid state luminophores is directly proportional to the intermolecular charge transfer intensity
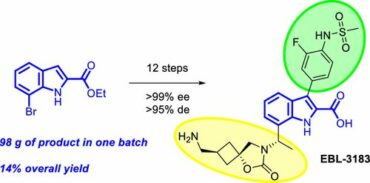
44. Optimized Synthesis of Indole Carboxylate Metallo-β-Lactamase Inhibitor EBL-3183
Baran, A.; Kuzmins, J.; Kuznecovs, J.; Farley, A., J., M.; Panduwawala, T.; Parkova, A.; Donets, P., A.; Brem, J.; Suna, E.; Schofield, C., J.; Shubin, K. Org. Process Res. Dev. 2023, 27, 692–706. DOI:10.1021/acs.oprd.3c00002