Heterocycles are among the most frequently encountered scaffolds in drugs and pharmaceutically relevant substances. Among the plethora of available methods for their functionalization, the most appealing are those relying on a transition metals-catalyzed direct transformation of the C–H to C–C or C–Heteroatom bonds.
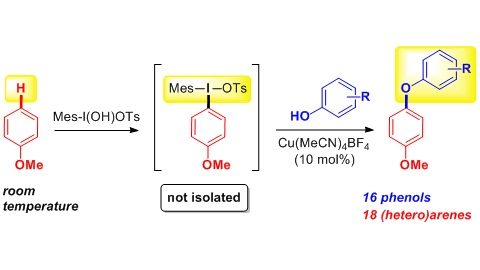
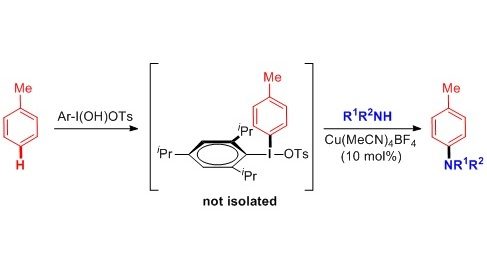
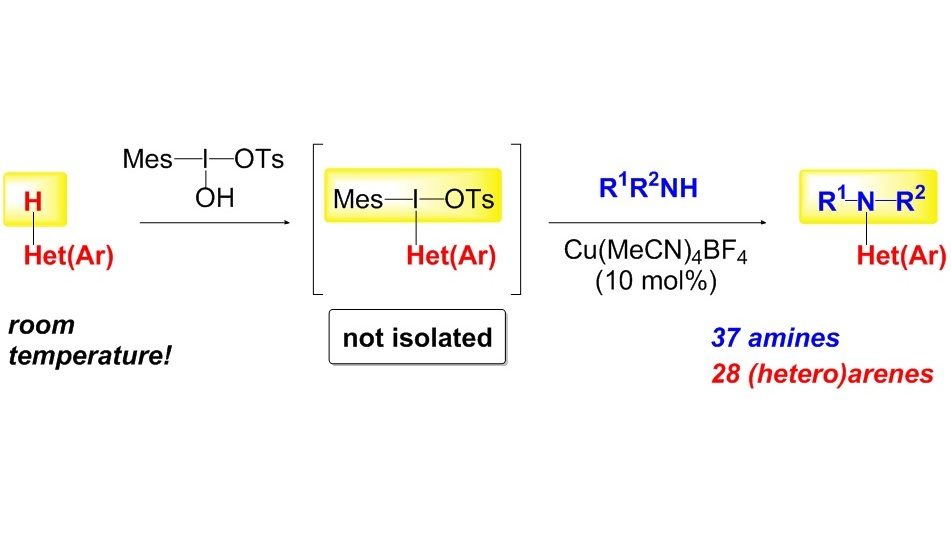
We have developed one-pot sequential multi-step approach to functionalization of aromatic and heterocyclic C–H bonds. The methodology comprises an initial reaction of electron-rich arenes or heteroarenes with a suitable I(III) reagent. The in situ formed unsymmetrical diaryl-λ3-iodanes subsequently undergo Cu or Pd-catalyzed reaction with wide range of oxygen and nitrogen nucleophiles.
Funding
Latvian Science Council Grant No. 274/2012
Publications
16. Copper-Catalyzed para–Selective C–H Amination of Electron-Rich Arenes
Berzina, B.; Sokolovs, I.; Suna, E. ACS Catalysis 2015, 5, 7008–7014.
12. Copper-Catalyzed Intermolecular C-H Amination of (Hetero)arenes via Transient Unsymmetrical lambda3-Iodanes
Sokolovs, I.; Lubriks, D.; Suna, E. J. Am. Chem. Soc. 2014, 136, 6920–6928.
DOI: 10.1021/ja502174d
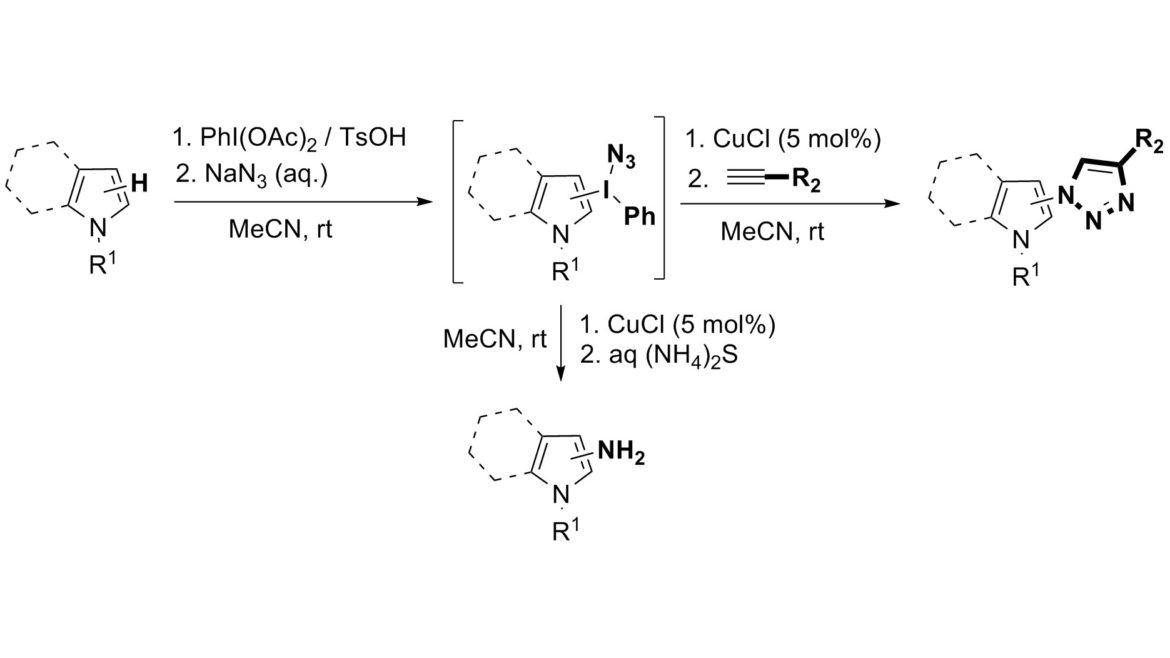
9. Indirect C–H Azidation of Heterocycles via Copper-Catalyzed Regioselective Fragmentation of Unsymmetrical λ3-Iodanes
Lubriks, D.; Sokolovs, I.; Suna, E. J. Am. Chem. Soc. 2012, 134, 15436-15442.
DOI: 10.1021/ja305574k
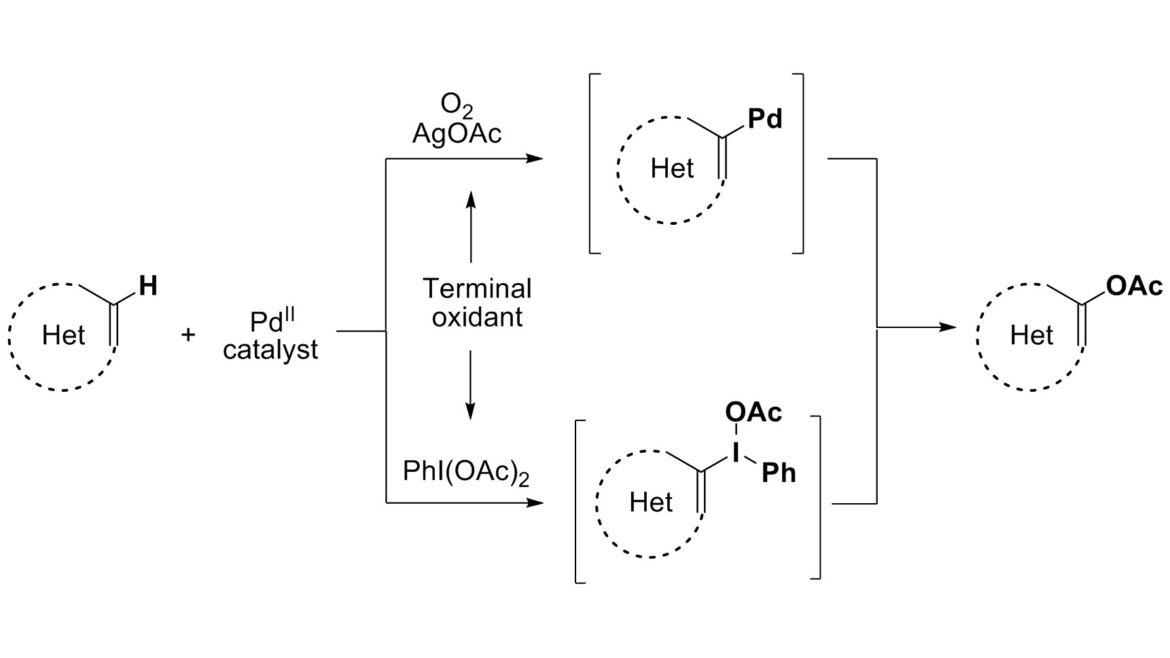
8. Transition-Metal-Catalyzed Acetoxylation of Heterocycles: All that Glitters is not Palladium (Highlight)
Suna, E. Chem. Heterocycl. Comp. 2012, 48, 44-48.
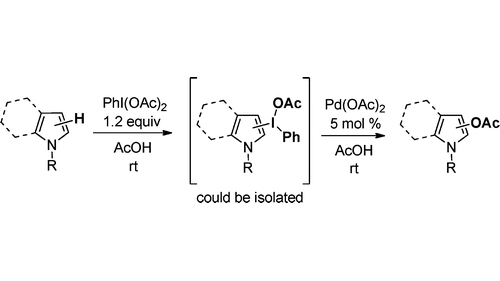
7. Iodonium Salts Are Key Intermediates in Pd-Catalyzed Acetoxylation of Pyrroles
Lubriks, D.; Sokolovs, I.; Suna, E. Org. Lett. 2011, 13, 4324–4327.
DOI: 10.1021/ol201665c
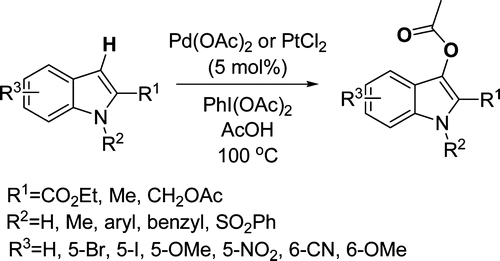
4. Catalytic Direct Acetoxylation of Indoles
Mutule, I.; Suna, E.; Olofsson, K.; Pelcman, B. J. Org. Chem. 2009, 74, 7195-7198.
DOI: 10.1021/jo901321b