Many pharmacologically relevant chemical compounds contain chiral amine functionality. Therefore the development of stereoselective methods for synthesis of chiral, non-racemic amines is of high importance in organic and pharmaceutical chemistry. Stereoselective synthesis employing Ellman’s chiral tert-butanesulfinamide reagent is among the most efficient ways to prepare chiral amines.
We are interested in the application of Ellman’s N-tert-butanesulfinyl chiral auxiliary in the development of new synthetic methods to access amines with multiple stereogenic centers.
Publications
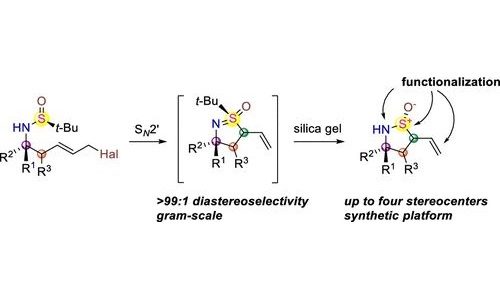
39. Synthetic Approach toward Enantiopure Cyclic Sulfinamides
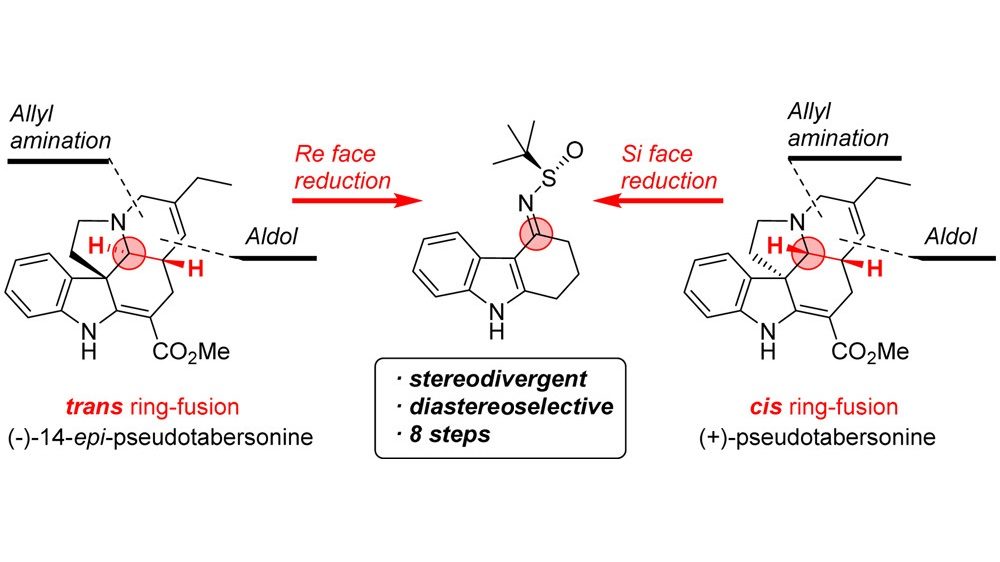
20. Stereodivergent Synthesis of Pseudotabersonine Alkaloids
Kazak, M.; Priede, M.; Shubin, K.; Bartrum, H. E.; Poisson, J.-F.; Suna, E. Org. Lett. 2017, 19, 5356–5359.
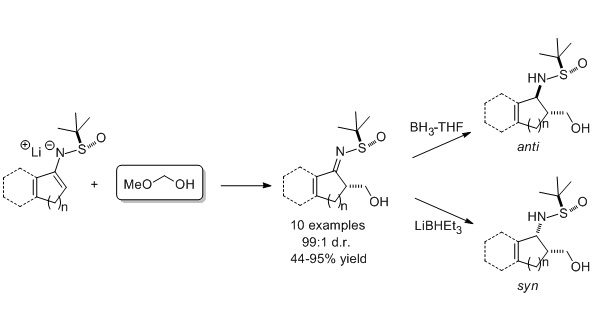
11. Diastereoselective Hydroxymethylation of Cyclic N–tert-Butanesulfinylketimines Using Methoxymethanol as Formaldehyde Source
Priede, M.; Kazak, M.; Kalnins, T.; Shubin, K.; Suna, E. J. Org. Chem. 2014, 79, 3715-3724.
DOI: 10.1021/jo500506u
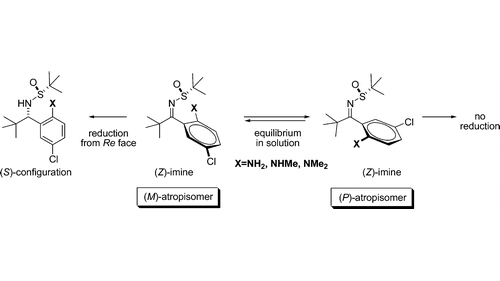
6. Asymmetric Synthesis of 1,3-Diamines. II: Diastereoselective Reduction of Atropisomeric N–tert-Butanesulfinylketimines
Martjuga, M.; Belyakov, S.; Liepinsh, E.; Suna, E. J. Org. Chem. 2011, 76, 2635–2647.
DOI: 10.1021/jo1025767
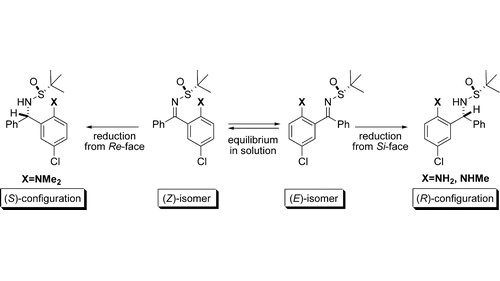
5. Asymmetric Synthesis of 1,3-Diamines by Diastereoselective Reduction of Enantiopure N–tert-Butanesulfinylketimines: Unusual Directing Effects of the ortho-Substituent
Martjuga, M.; Shabashov, D.; Belyakov, S.; Liepinsh, E.; Suna, E. J. Org. Chem. 2010, 75, 2375-2368.
DOI: 10.1021/jo100173f