Organic electrosynthesis is inherently environmentally benign technique because it uses the electron as a redox agent rather than chemical oxidants or reductants as in traditional chemistry. As such, electrosynthesis does not produce waste. The use of redox mediators to achieve indirect electrolysis offers many advantages as compared to a direct electrolysis. For example, electron transfer can occur against a potential gradient, allowing for electrolysis at lower potentials and reducing the undesired side-reactions. In addition, the use of electron transfer mediators can help to avoid electrode passivation resulting from polymer film formation on the electrode surface.
We are interested in the design and use of hypervalent I(III) species as a redox mediators.
Publications
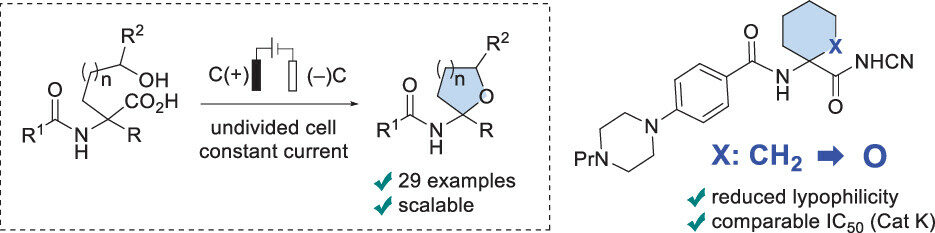
47. Electrochemical Synthesis of Unnatural Amino Acids via Anodic Decarboxylation of N-Acetylamino Malonic Acid Derivatives
DOI: https://doi.org/10.1021/acs.orglett.3c02687.
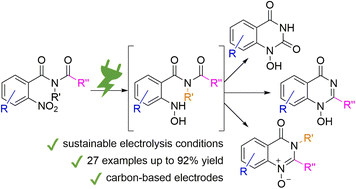
42. Simple and scalable electrosynthesis of 1H-1-hydroxy-quinazolin-4-ones
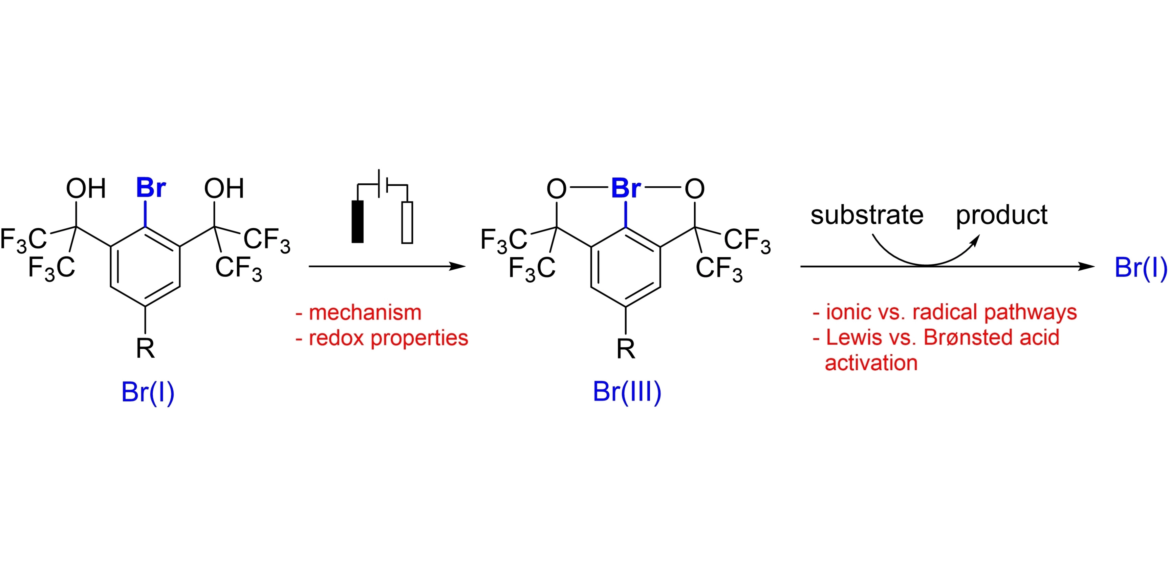
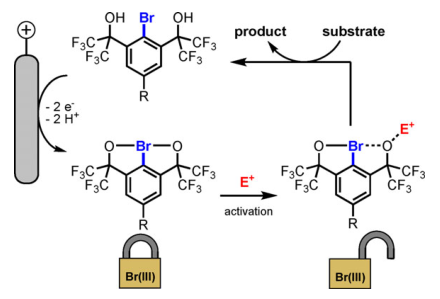
40. Electrochemistry and Reactivity of Chelation-stabilized Hypervalent Bromine(III) Compounds
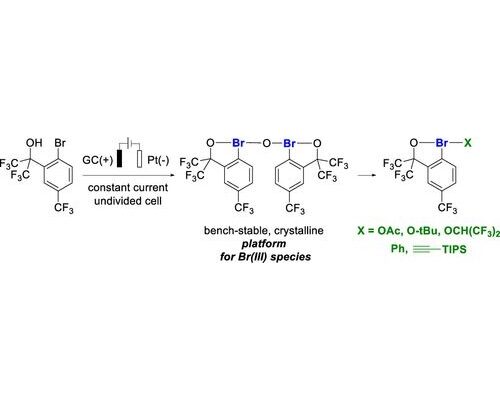
34. Electrochemical Generation of Hypervalent Bromine(III) Compounds
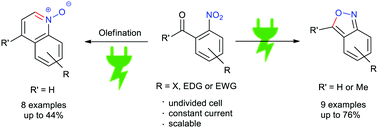
26. Simple and scalable electrochemical synthesis of 2,1-benzisoxazoles and quinoline N-oxides
DOI: 10.1039/C9CC06054E
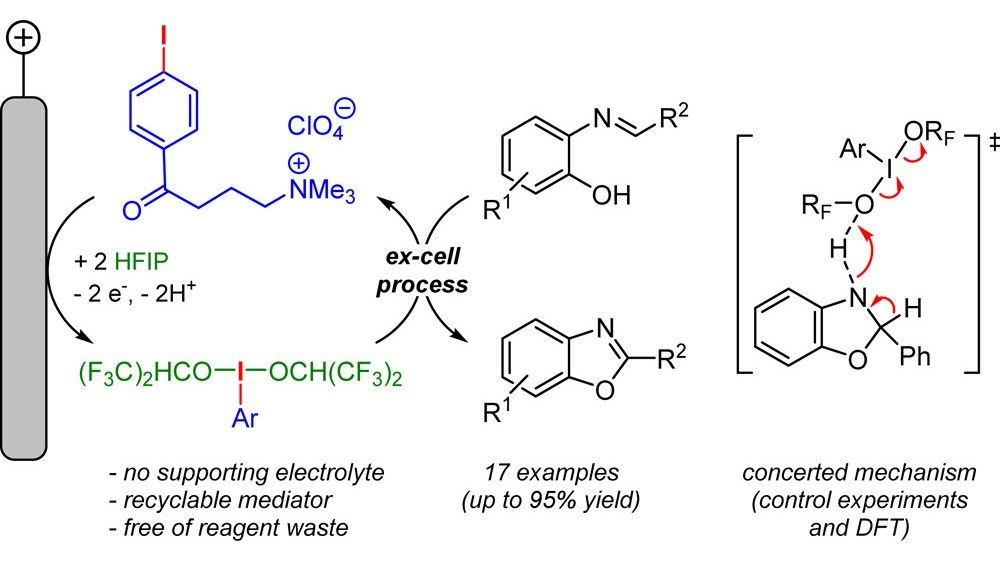
19. Synthesis of Benzoxazoles Using Electrochemically Generated Hypervalent Iodine
Koleda, O.; Broese, T.; Noetzel, J.; Roemelt, M.; Suna, E.; Francke, R. J. Org. Chem. 2017, 82, 11669–11681.