Vitkovska, V.; Zogota, R.; Kalnins, T.; Zelencova, D.; Suna, E. Chem. Heterocycl. Comp. 2020, 56, 586–602.
DOI: 10.1007/s10593-020-02704-6
Abstract
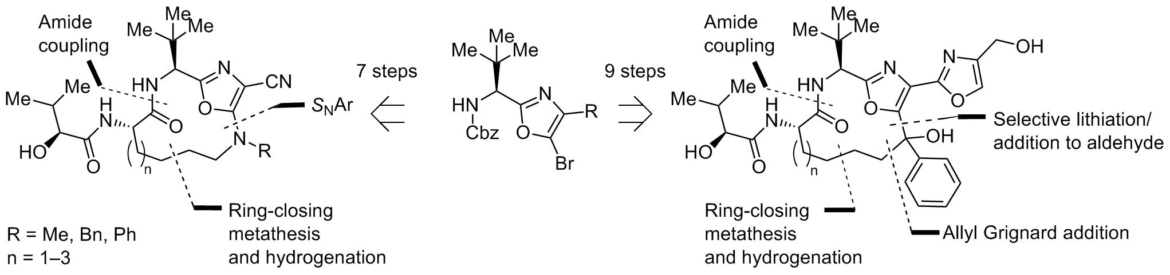
Aliphatic alkyl chain-containing 12–14-membered macrocycles have been designed as structural analogs of antimitotic natural product diazonamide A. Macrocycles were synthesized from 5-bromooxazole in 7 to 9 linear steps using Ru-catalyzed ring-closing metathesis as the key transformation. Heat effect of binding to α,β-tubulin tetramer (T4-RB3 complex) has been measured for the synthesized macrocycles by isothermal titration calorimetry method.
Date: Jun 13, 2020
AUTHOR: Artis Kinens