Martjuga, M.; Belyakov, S.; Liepinsh, E.; Suna, E. J. Org. Chem. 2011, 76, 2635–2647.
DOI: 10.1021/jo1025767
Abstract
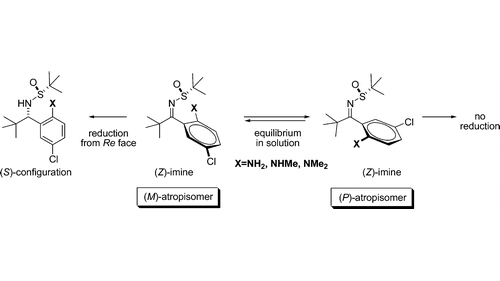
Chiral, nonracemic o-aminobenzylamines were prepared in a highly diastereoselective reduction of atropisomeric N–tert-butanesulfinylketimines. The ortho-substituent ensures the distinct reactivity of atropisomers 4d−f. The free energy of activation for atropisomerization of sulfinylimines 4d−f in THF-d8 was determined by NMR methods to range from 70.8 to 97.9 kJ/mol.
Date: Mar 16, 2011
AUTHOR: Artis Kinens