Martjuga, M.; Shabashov, D.; Belyakov, S.; Liepinsh, E.; Suna, E. J. Org. Chem. 2010, 75, 2375-2368.
DOI: 10.1021/jo100173f
Abstract
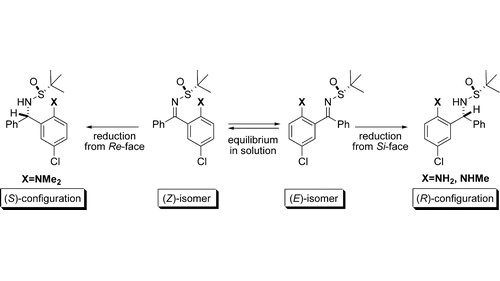
Chiral, nonracemic 1,3-diamines were prepared in a highly diastereoselective reduction of diaryl N–tert-butanesulfinylketimines. Correlation between facial selectivity of the reduction and E or Z geometry of the starting ketimines suggests involvement of a cyclic transition state for the reduction. The ortho-substituent controls the geometry of N–tert-butanesulfinylketimines in the solid state and provides additional stabilization of the cyclic transition state.
Date: Mar 3, 2010
AUTHOR: Artis Kinens