Kluga R., Kinens A., Suna E. Chem. Eur. J. 2024, e202301136
DOI: 10.1002/chem.202301136.
Abstract
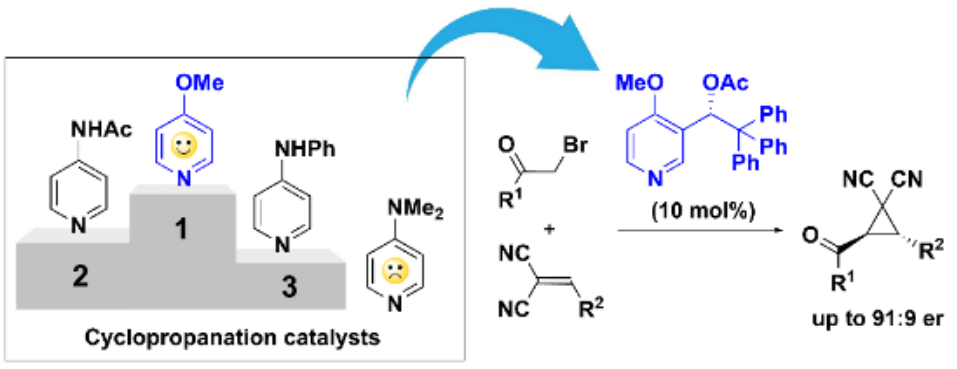
The design of pyridine–derived organocatalysts aims at the increase of their Lewis basicity, however such an approach is not always efficient. For example, strongly Lewis basic DMAP is completely inefficient as catalyst in the cyclopropanation reaction. Herein we disclose an alternative approach that relies on attenuation of DMAP Lewis basicity. Specifically, the replacement of 4–dimethylamino substituent in DMAP for 4–MeO group delivered a highly efficient catalyst for cyclopropanation of electron–deficient olefins with α-bromoketones. Kinetic studies provide compelling evidence that the superior catalytic efficiency of 4–MeO pyridine (MOPY) is to be attributed to the favorable balance between Lewis basicity and leaving group ability. The use of chiral, enantiomerically pure MOPY catalyst has helped to achieve high enantioselectivities (up to 91:9 er) in the previously unreported pyridine–catalyzed cyclopropanation reaction.