Priede, M.; Kazak, M.; Kalnins, T.; Shubin, K.; Suna, E. J. Org. Chem. 2014, 79, 3715-3724.
DOI: 10.1021/jo500506u
Abstract
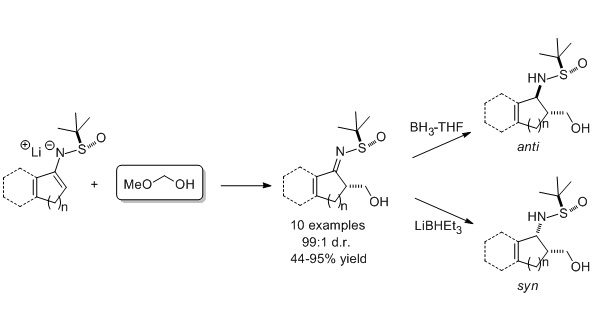
Hydroxymethylation of cyclic tert-butanesulfinylketimine-derived lithium enamides with methoxymethanol proceeds with excellent diastereoselectivity (99:1 dr). Methoxymethanol is a stable and easy-to-handle source of anhydrous monomeric formaldehyde in the reaction with lithium enamides. Cyclic α-hydroxymethyl ketimines undergo highly diastereoselective reduction to syn– or anti-1,3-amino alcohols.
Date: Mar 25, 2014
AUTHOR: Artis Kinens