Sokolovs, I.; Mohebbati, N.; Francke, R.; Suna, E. Angew. Chem. Int. Ed. 2021, 60, 15832–15837. Download publication.
Abstract
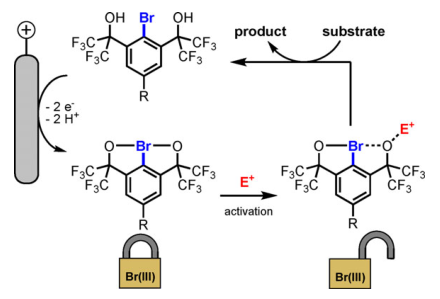
In sharp contrast to hypervalent iodine(III) compounds, the isoelectronic bromine(III) counterparts have been little studied to date. This knowledge gap is mainly attributed to the difficult-to-control reactivity of λ3-bromanes as well as to their challenging preparation from the highly toxic and corrosive BrF3 precursor. In this context, we present a straightforward and scalable approach to chelation-stabilized λ3-bromanes by anodic oxidation of parent aryl bromides possessing two coordinating hexafluoro-2-hydroxypropanyl substituents. A series of para-substituted λ3-bromanes with remarkably high redox potentials spanning a range from 1.86 V to 2.60 V vs. Ag/AgNO3 was synthesized by the electrochemical method. We demonstrate that the intrinsic reactivity of the bench-stable bromine(III) species can be unlocked by addition of a Lewis or a Brønsted acid. The synthetic utility of the λ3-bromane activation is exemplified by oxidative C−C, C−N, and C−O bond forming reactions.