Lubriks, D.; Sokolovs, I.; Suna, E. J. Am. Chem. Soc. 2012, 134, 15436-15442.
DOI: 10.1021/ja305574k
Abstract
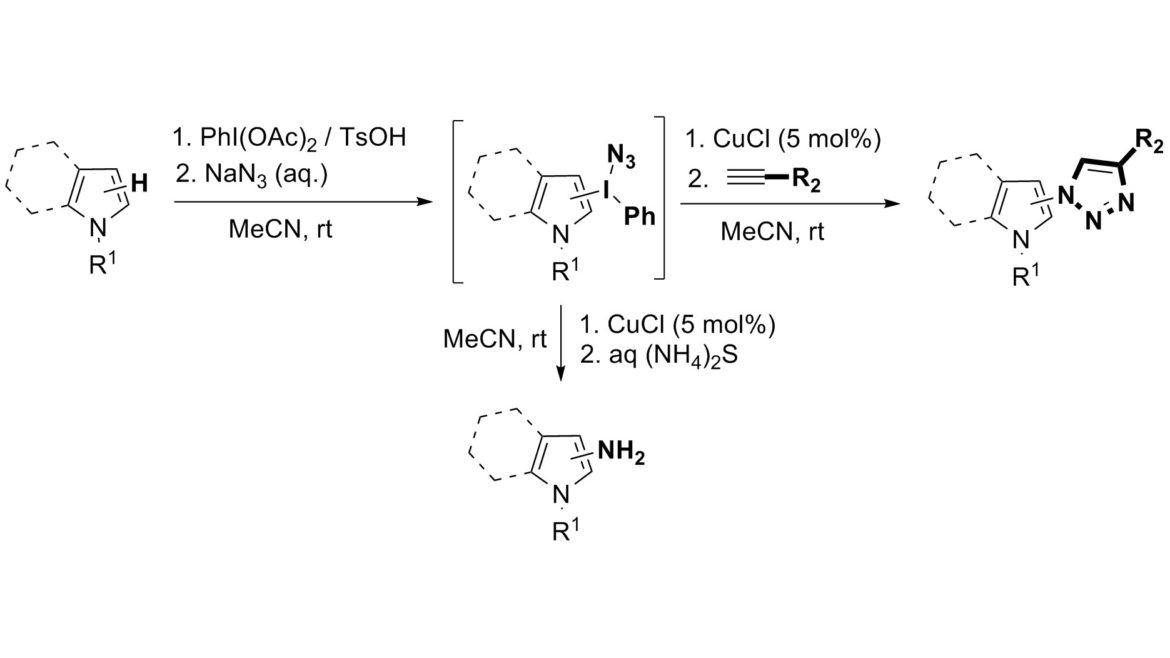
A C–H bond of electron-rich heterocycles is transformed into a C–N bond in a reaction sequence comprising the formation of heteroaryl(phenyl)iodonium azides and their in situ regioselective fragmentation to heteroaryl azides. A Cu(I) catalyst ensures complete regiocontrol in the fragmentation step and catalyzes the subsequent 1,3-dipolar cycloaddition of the formed azido heterocycles with acetylenes. The heteroaryl azides can also be conveniently reduced to heteroarylamines by aqueous ammonium sulfide. The overall C–H to C–N transformation is a mild and operationally simple one-pot sequential multistep process.
Date: Aug 22, 2012
AUTHOR: Artis Kinens