Krasikovs, A; Ozola, V.; Dax, S. L.; Suna, E. Synthesis 2013, 45, 683-693.
Abstract
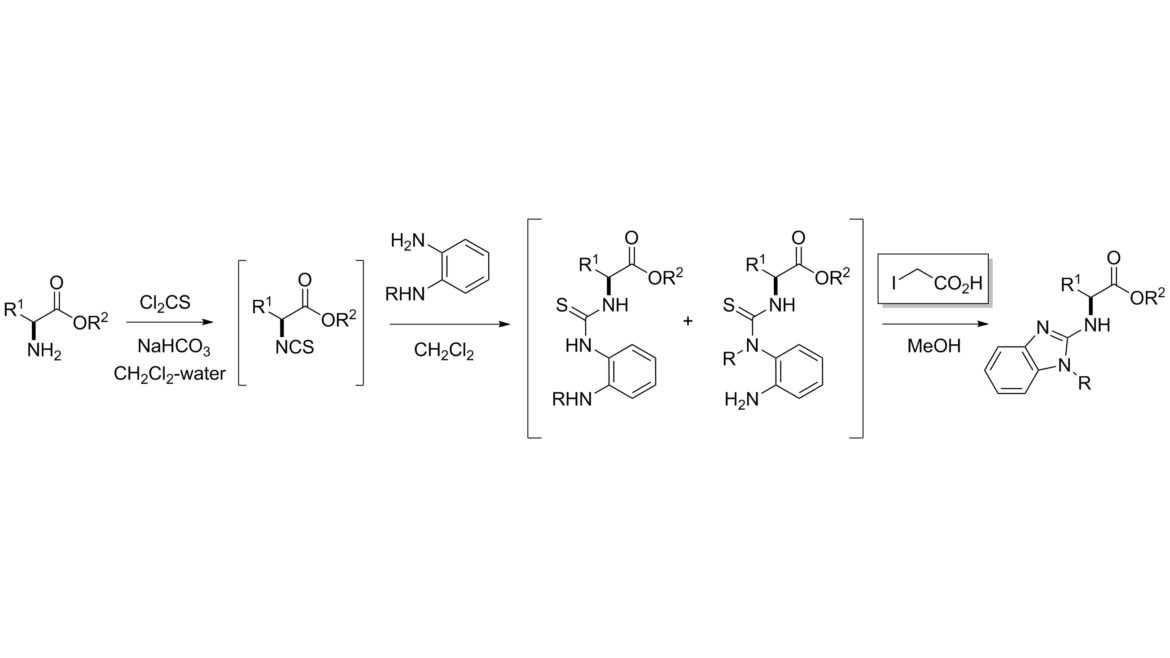
Chiral, nonracemic, N-unprotected amino acids were converted into the corresponding N-benzimidazol-2-yl derivatives by a sequential procedure involving initial formation of isothiocyanates, their reaction with arene-1,2-diamines, and cyclization–desulfurization of the intermediate thioureas with iodoacetic acid. The simplified workup and the lack of volatile or toxic byproducts in the key desulfurization step renders iodoacetic acid a superior reagent to the usual reagent, iodomethane.
Date: Feb 21, 2013
AUTHOR: Artis Kinens