Koleda O., Prane K., Suna E. Org. Lett. 2023, 25 (44), 7958–7962.
DOI: https://doi.org/10.1021/acs.orglett.3c02687.
Abstract
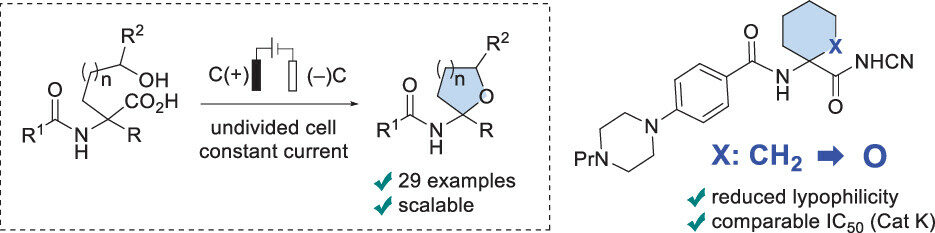
Broad application of α,α-disubstituted cyclic amino acid derivatives in medicinal chemistry urges for analogue design with improved pharmacokinetic properties. Herein, we disclose an electrochemical approach toward unnatural THF- and THP-containing amino acid derivatives that relies on anodic decarboxylation-intramolecular etherification of inexpensive and readily available N-acetylamino malonic acid monoesters under Hofer–Moest reaction conditions. The decarboxylative cyclization proceeds under constant current conditions in an undivided cell in an aqueous medium without any added base. A successful bioisosteric replacement of the 1-aminocyclohexane-1-carboxylic acid subunit by the THP-containing amino acid scaffold in cathepsin K inhibitor balicatib helped to reduce lipophilicity while retaining low nanomolar enzyme inhibitory potency and comparable microsomal stability.