Baran, A.; Kuzmins, J.; Kuznecovs, J.; Farley, A., J., M.; Panduwawala, T.; Parkova, A.; Donets, P., A.; Brem, J.; Suna, E.; Schofield, C., J.; Shubin, K. Org. Process Res. Dev. 2023, 27, 692–706. DOI:10.1021/acs.oprd.3c00002
Abstract
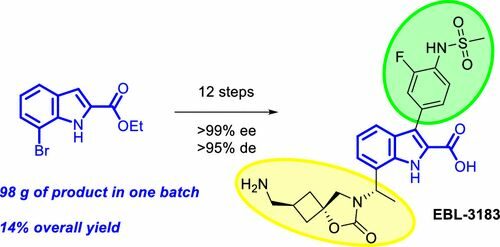
A new synthetic route for the preparation of the metallo-β-lactamase inhibitor pre-candidate EBL-3183 was developed and carried out on a kilogram scale. The described process starts from a commercially available indole-2-carboxylate and employs an Ellman auxiliary approach coupled with ruthenium-catalyzed stereoselective reduction for the introduction of chirality. The key spirocyclic cyclobutane motif was assembled utilizing an epoxide building block, which was conveniently obtained in diastereomerically pure form. The amount and quality of the prepared final target EBL-3183 were sufficient for the preclinical studies.