Kazak, M.; Vasilevska, A.; Suna, E. Chem. Heterocycl. Comp. 2020, 56, 355–364.
DOI: 10.1007/s10593-020-02667-8
Abstract
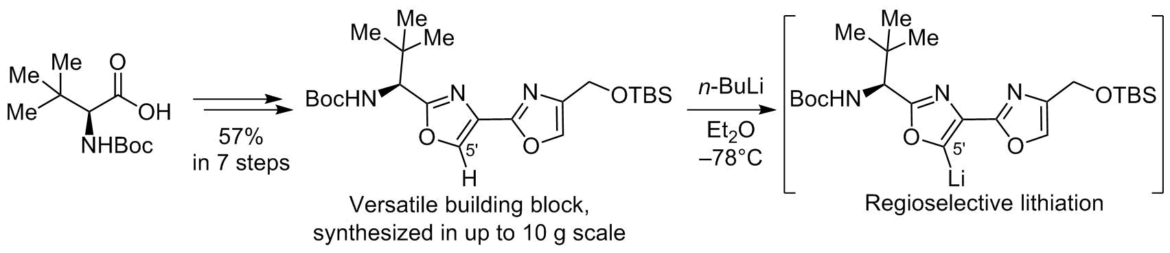
Seven-step preparative scale synthesis of chiral, enantiomerically pure 2′-aminomethyl-2,4′-bioxazole has been accomplished from L-tert-Leu. The 2,4′-bioxazole subunit can be functionalized at position C-5′ by a regioselective lithiation, followed by the reaction with a suitable electrophile. The C-5′-lithium intermediate can be also transmetalated into organozinc species and used in Pd-catalyzed Negishi cross coupling.
Date: Jun 13, 2020
AUTHOR: Artis Kinens