Rodrigo, E.; Baunis, H.; Suna, E.; Waldvogel, S., R. Chem. Commun., 2019, 55, 12255-1225.
DOI: 10.1039/C9CC06054E
Abstract
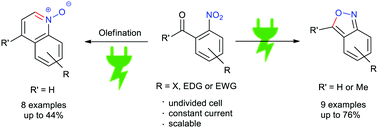
Cathodic reduction of the nitro moiety and subsequent intramolecular cyclization affords different substituted 2,1-benzisoxazoles and quinoline N-oxides. This methodology allows the synthesis of two different types of heterocycles from common simple starting materials, using electrons as a sole reagent for this transformation. The electrolysis can be conducted in a very simple undivided electrolysis cell under constant current conditions. This permits working on a larger scale compared to other electrochemical methodologies and represents a significant advantage.
Date: Oct 15, 2019
AUTHOR: Artis Kinens