Lubriks, D.; Haldimann, K.; Kiliç, F.; Hartmann, M.; Böttger, E. C.; Hobbie, S. N.; Suna, E.; Crich, D. Helv. Chim. Acta 2023, e202300138
DOI: 10.1002/hlca.202300138.
Abstract
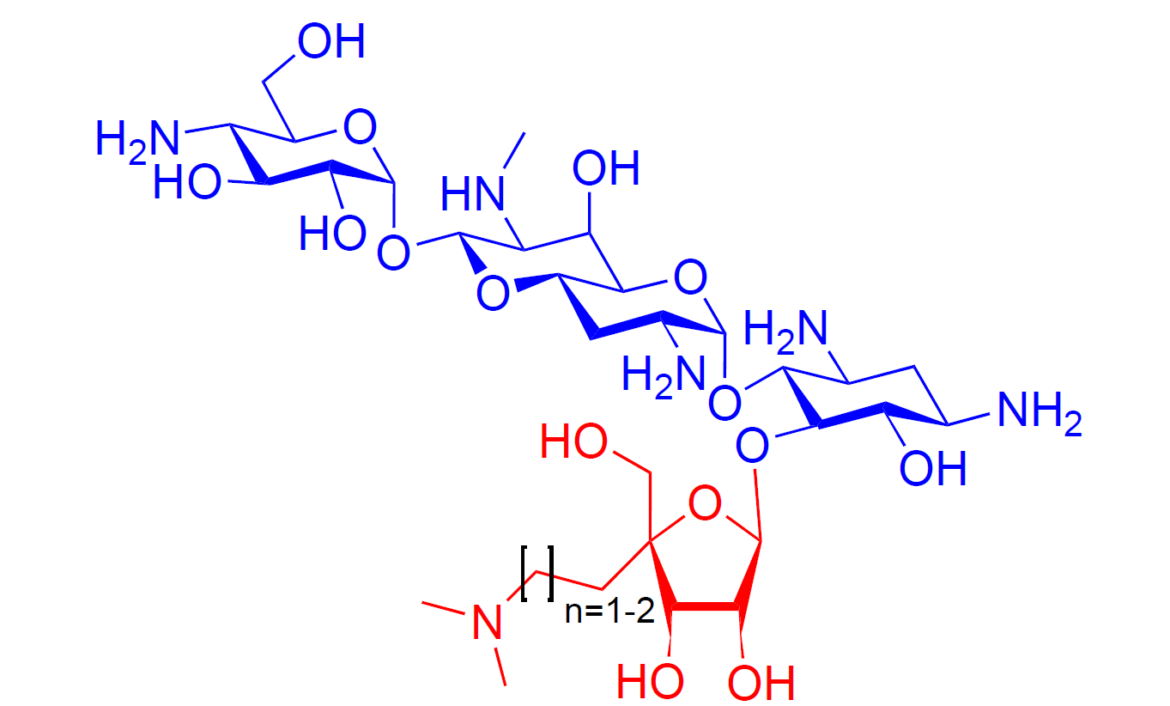
We report the synthesis and evaluation of two new apramycin 5-O-β-d-ribofuranosides, or apralogs, carrying aminoalkyl branches at the ribofuranose 4-position. This novel modification conveys excellent activity for the inhibition of protein synthesis by wild-type bacterial ribosomes and correspondingly high antibacterial activity against several Gram-negative pathogens. Notably, these new modifications overcome the reduction of antibacterial activity in other 2-deoxystreptamine-type aminoglycosides carrying a 5-O-ribofuranosyl moiety when challenged by the presence of an aminoglycoside phosphotransferase enzyme capable of acting on the ribose 5-position.