Jersovs, G.; Bojars, M.; Donets, P., A.; Suna, E. Org. Lett. 2022, 24, 4625–4629.
DOI: 10.1021/acs.orglett.2c01738
Abstract
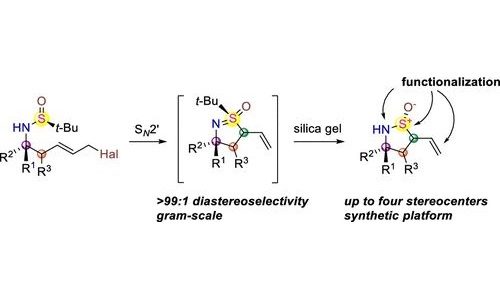
A synthetic approach toward densely substituted enantiopure cyclic sulfinamides possessing up to four consecutive stereogenic centers was developed based on a completely diastereoselective SN2′ cyclization/tert-Bu cleavage sequence. Diastereospecific transformation of the obtained scaffold into chiral SVI derivatives such as sulfoximines and sulfonimidamides is demonstrated.
Date: Jun 17, 2022
AUTHOR: Artis Kinens